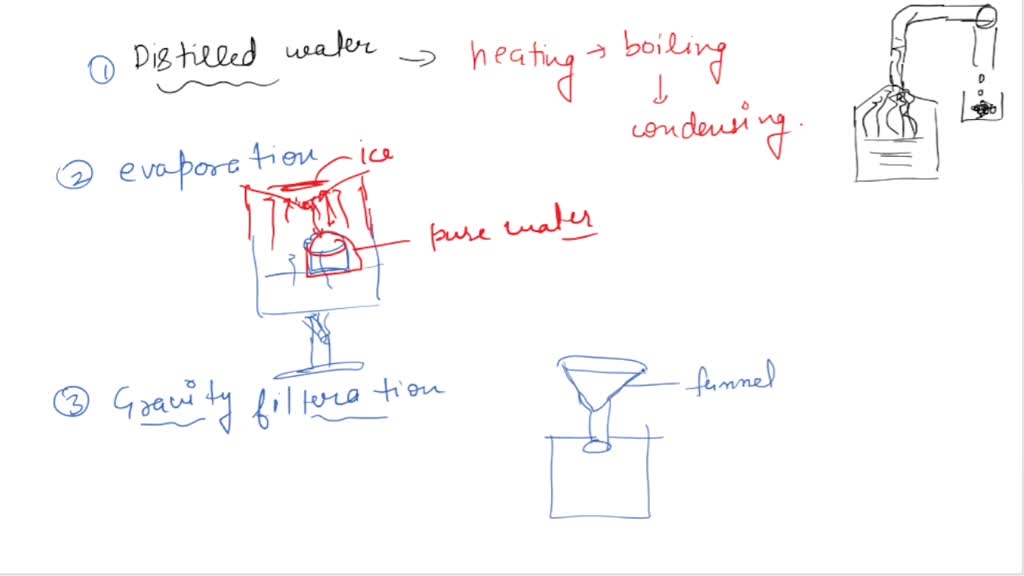
Filtration Evaporation Distillation . In simple distillation, a mixture is heated, and the most volatile component vaporizes at the lowest temperature. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. let's learn about some of the most commonly used methods of separation such as handpicking, sieving, evaporation,. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or. for evaporation and distillation, pure substances have specific boiling/condensation points and melting/freezing points.
from www.numerade.com
In simple distillation, a mixture is heated, and the most volatile component vaporizes at the lowest temperature. For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. for evaporation and distillation, pure substances have specific boiling/condensation points and melting/freezing points. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. let's learn about some of the most commonly used methods of separation such as handpicking, sieving, evaporation,. Distillation is an effective method to separate mixtures comprised of two or more pure liquids.
SOLVED Distillation, evaporation, gravity filtration, and vacuum
Filtration Evaporation Distillation there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or. for evaporation and distillation, pure substances have specific boiling/condensation points and melting/freezing points. For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. let's learn about some of the most commonly used methods of separation such as handpicking, sieving, evaporation,. In simple distillation, a mixture is heated, and the most volatile component vaporizes at the lowest temperature.
From www.youtube.com
Separation of mixtures, sublimation, filtration, evaporation Filtration Evaporation Distillation there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or. For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. let's learn about some of the most commonly used methods of separation such as handpicking, sieving, evaporation,. Distillation is a purification process where one component of a. Filtration Evaporation Distillation.
From www.slideserve.com
PPT SEPARATION TECHNIQUES PowerPoint Presentation, free download ID Filtration Evaporation Distillation there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. for evaporation and distillation, pure substances have specific boiling/condensation points and melting/freezing points. distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. Distillation is a purification process where one component of a liquid mixture. Filtration Evaporation Distillation.
From www.numerade.com
SOLVED Distillation, evaporation, gravity filtration, and vacuum Filtration Evaporation Distillation For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. In simple distillation, a mixture is heated, and the most volatile component vaporizes at the lowest temperature. Distillation is an effective method. Filtration Evaporation Distillation.
From www.tes.com
Evaporation and distillation KS3 Activate Science Teaching Resources Filtration Evaporation Distillation Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. let's learn about some of the most commonly used methods of separation such as handpicking, sieving, evaporation,. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or. For filtration, crystallisation and chromatography, different substances have different. Filtration Evaporation Distillation.
From dxowdbrdd.blob.core.windows.net
Evaporation And Distillation Are Used To Separate The Contents Of A at Filtration Evaporation Distillation In simple distillation, a mixture is heated, and the most volatile component vaporizes at the lowest temperature. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. for evaporation and distillation, pure substances have specific boiling/condensation points and melting/freezing points. Distillation is an effective method to separate mixtures comprised of. Filtration Evaporation Distillation.
From www.slideserve.com
PPT SEPARATION TECHNIQUES PowerPoint Presentation, free download ID Filtration Evaporation Distillation For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. for evaporation and distillation, pure substances have specific boiling/condensation points and melting/freezing points. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or. let's learn about some of the most commonly used methods of separation such. Filtration Evaporation Distillation.
From dxondimvq.blob.core.windows.net
Filtration And Distillation Method at Mary Bowser blog Filtration Evaporation Distillation there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. In simple distillation, a mixture is heated, and the most volatile component. Filtration Evaporation Distillation.
From www.youtube.com
Evaporation and Distillation Mr H YouTube Filtration Evaporation Distillation Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. Distillation is an. Filtration Evaporation Distillation.
From www.youtube.com
Separating Mixtures, Different Methods Distillation, Evaporation Filtration Evaporation Distillation Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. Distillation is an. Filtration Evaporation Distillation.
From www.scribd.com
Name The Separation Technique Shown in The Diagram. A. Evaporation B Filtration Evaporation Distillation For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. In simple distillation, a mixture is heated, and the most volatile component vaporizes at the lowest temperature. distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. let's learn about some. Filtration Evaporation Distillation.
From www.youtube.com
How to separate Mixtures filtration, distillation, sublimation and Filtration Evaporation Distillation there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or. let's learn about some of the most commonly used methods of separation such as handpicking, sieving, evaporation,. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. distillation is a purification process where the components of a liquid mixture are. Filtration Evaporation Distillation.
From dxondimvq.blob.core.windows.net
Filtration And Distillation Method at Mary Bowser blog Filtration Evaporation Distillation In simple distillation, a mixture is heated, and the most volatile component vaporizes at the lowest temperature. distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. For filtration, crystallisation and chromatography, different substances have. Filtration Evaporation Distillation.
From www.slideserve.com
PPT SEPARATION TECHNIQUES PowerPoint Presentation, free download ID Filtration Evaporation Distillation In simple distillation, a mixture is heated, and the most volatile component vaporizes at the lowest temperature. distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. there are different ways to separate mixtures, eg. Filtration Evaporation Distillation.
From www.scribd.com
Separating Mixtures Filtration, Evaporation, Distillation, and Filtration Evaporation Distillation distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. for evaporation and distillation, pure substances have specific boiling/condensation points and melting/freezing points. there are different ways to separate mixtures,. Filtration Evaporation Distillation.
From www.geeksforgeeks.org
Methods of Separation Various Separation Techniques Filtration Evaporation Distillation For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. distillation is a purification process where the. Filtration Evaporation Distillation.
From www.dreamstime.com
Separating by Filtration and Evaporation Condensation the Component Filtration Evaporation Distillation there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or. for evaporation and distillation, pure substances have specific boiling/condensation points and melting/freezing points. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. distillation is. Filtration Evaporation Distillation.
From www.tffn.net
How Does Distillation Work? A Comprehensive Guide The Enlightened Mindset Filtration Evaporation Distillation For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. In simple distillation, a mixture is heated, and the most volatile component vaporizes at the lowest temperature. Distillation is an effective method to. Filtration Evaporation Distillation.
From 12014dorcascharityng.weebly.com
FILTRATION,EVAPORATION,DISTILLATION AND PAPER CHROMATOGRAPHY 3E1 Filtration Evaporation Distillation for evaporation and distillation, pure substances have specific boiling/condensation points and melting/freezing points. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. For filtration, crystallisation and chromatography, different substances have different solubilities in the same solvent at a given temperature. distillation is a purification process where the components of a liquid mixture are. Filtration Evaporation Distillation.